CyPath® Lung sales up 276% year-over-year
SAN ANTONIO, Texas (May 15, 2025) – bioAffinity Technologies, Inc. (Nasdaq: BIAF; BIAFW), a biotechnology company focused on the need for accurate, noninvasive tests for the detection of early-stage lung cancer and other lung diseases, today reported financial results for the three months ended March 31, 2025.
Key Highlights
- Generated revenue of $1.9 million in the first quarter of 2025.
- CyPath® Lung sales up 276% year-over-year in the first quarter of 2025.
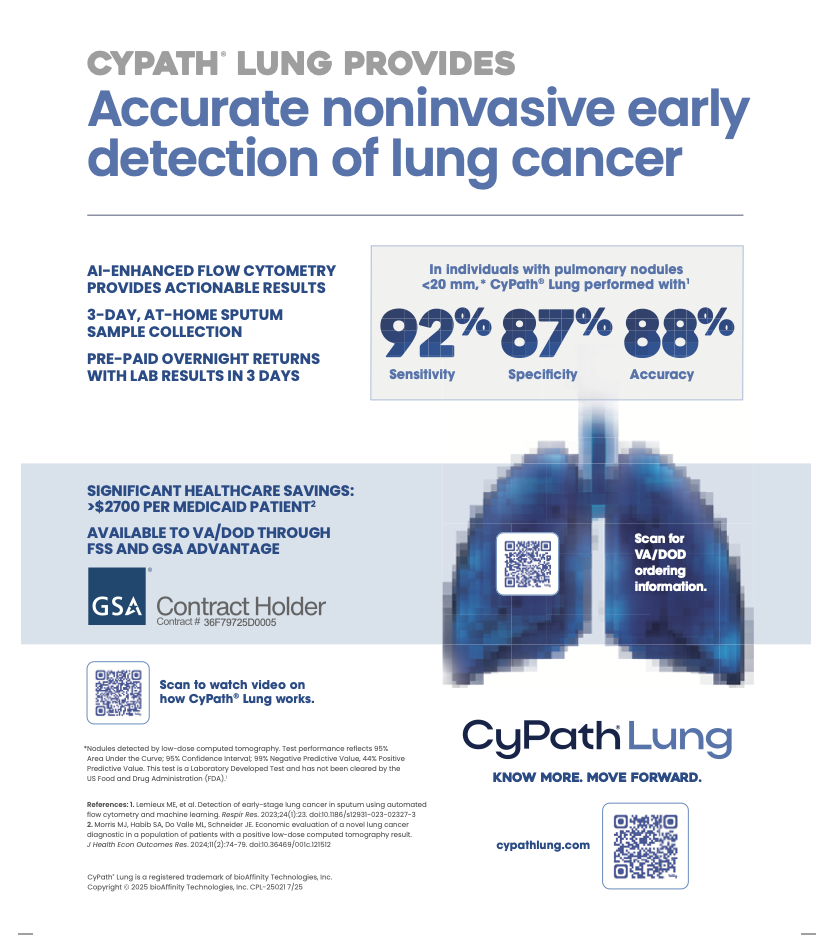
- Reported strong results from the Texas beta launch of CyPath® Lung, with first-quarter test sales building on the momentum of more than 600 tests delivered in 2024.
- Announced strategic actions expected to reduce annual costs by approximately $3.8 million while expanding sales focus on high-margin diagnostics like CyPath® Lung.
- Released multiple patient case studies highlighting CyPath® Lung’s impact in real-world clinical settings, including early detection of second primary lung cancer and avoidance of unnecessary invasive procedures.
- Received acceptance of a new patent application from the Australian Patent Office, strengthening international IP protection for CyPath® Lung and expanding global commercialization potential.
- Subsequent to the end of the first quarter, announced improvements to CyPath® Lung processing that increased data acquisition throughput by 50% and reduced unit cost by more than 25%, enhancing efficiency without compromising test performance.
Management Commentary
“We began 2025 with strong momentum, delivering 276% year-over-year growth in CyPath® Lung sales and expanding our commercial footprint across the U.S.,” said Maria Zannes, President and Chief Executive Officer of bioAffinity Technologies. “Our first-quarter results reflect both the growing demand for our noninvasive lung cancer diagnostic and the successful execution of our strategy to focus on high-value services and operational efficiency.
“During the quarter, we took decisive actions to streamline operations at our subsidiary lab by reducing labor costs, implementing operational efficiencies and discontinuing certain pathology services with suboptimal profit margins. These targeted actions will reduce costs approximately $3.8 million annually and accelerate the commercial growth of CyPath® Lung. These changes are already translating into margin improvements and enhanced resource allocation in support of our commercialization goals.
“In parallel, patient case studies continue to underscore the diagnostic power of CyPath® Lung in real-world settings — from avoiding unnecessary invasive procedures to identifying secondary or recurrent cancers. As we move forward, we’re encouraged by the clinical and economic validation that supports our growth, including recent operational enhancements that have increased our test throughput by 50% and lowered per-test cost by over 25%.
“We remain committed to expanding access to CyPath® Lung for patients at risk of lung cancer and advancing new diagnostics for diseases like COPD and asthma,” Zannes added. “With every test delivered, we’re improving outcomes for patients and delivering value to our shareholders.”
First Quarter 2025 Financial Results
Revenue for the quarter ended March 31, 2025, was $1.9 million. Revenue was primarily generated from patient service fees, histology services, and medical director fees.
Operating expenses for the first quarter of 2025 were $4.5 million, compared with $4.4 million in the first quarter of 2024.
Direct costs and expenses for the first quarter of 2025 were $1.4 million, down 13% from $1.6 million in the prior-year period, primarily due to targeted strategic actions implemented in March 2025. Research and development expenses decreased 7% year-over-year to $367,000, reflecting lower employee compensation and lab supply costs. Clinical development expenses rose to $138,000, driven by higher professional fees supporting the Company’s pivotal clinical trial strategy.
Selling, general and administrative expenses were $2.5 million for the first quarter of 2025, up from $2.2 million in the same period last year. The increase was primarily driven by higher employee compensation related to administrative and sales functions, reflecting the addition of personnel and support services to scale the commercialization of CyPath® Lung.
Net loss for the quarter ended March 31, 2025, was $2.7 million, or $0.16 per share, compared with a net loss of $2.0 million, or $0.20 per share, for the first quarter of 2024.
Cash and cash equivalents as of March 31, 2025, were $0.4 million, compared with $1.1 million as of December 31, 2024. Subsequent to the end of 2024, bioAffinity Technologies raised gross proceeds of $1.4 million through the inducement of warrant exercises in February 2025 and $3.3 million in a public offering in May 2025.
About bioAffinity Technologies, Inc.
bioAffinity Technologies, Inc. addresses the need for noninvasive diagnosis of early-stage cancer and diseases of the lung and broad-spectrum cancer treatments. The Company’s first product, CyPath® Lung, is a noninvasive test that has shown high sensitivity, specificity and accuracy for the detection of early-stage lung cancer. CyPath® Lung is marketed as a Laboratory Developed Test (LDT) by Precision Pathology Laboratory Services, a subsidiary of bioAffinity Technologies. For more information, visit www.bioaffinitytech.com.
Forward-Looking Statements
Certain statements in this press release constitute “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “might,” “will,” “should,” “believe,” “expect,” “anticipate,” “estimate,” “continue,” “predict,” “forecast,” “project,” “plan,” “intend” or similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. These forward-looking statements are based upon current estimates and assumptions and include statements regarding announced strategic actions reducing annual costs by approximately $3.8 million while expanding sales focus on high-margin diagnostics like CyPath® Lung; international patents expanding CyPath® Lung’s global commercialization potential; the targeted actions accelerating the commercial growth of CyPath® Lung; patient case studies continuing to underscore the diagnostic power of CyPath® Lung in real-world settings; and expanding access to CyPath® Lung for patients at risk of lung cancer and advancing new diagnostics for diseases like COPD and asthma. These forward-looking statements are subject to various risks and uncertainties, many of which are difficult to predict that could cause actual results to differ materially from current expectations and assumptions from those set forth or implied by any forward-looking statements. Important factors that could cause actual results to differ materially from current expectations include, among others, the Company’s ability to reduce costs while expanding sales of high-margin diagnostics; the Company’s ability to accelerate the commercial growth of CyPath® Lung; the Company’s ability to advance new diagnostics for diseases like COPD and asthma; and the other factors discussed in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, and its subsequent filings with the SEC, including subsequent periodic reports on Forms 10-Q and 8-K. Such forward-looking statements are based on facts and conditions as they exist at the time such statements are made and predictions as to future facts and conditions. While the Company believes these forward-looking statements are reasonable, readers of this press release are cautioned not to place undue reliance on any forward-looking statements. The information in this release is provided only as of the date of this release, and the Company does not undertake any obligation to update any forward-looking statement relating to matters discussed in this press release, except as may be required by applicable securities laws
Contacts
bioAffinity Technologies
Julie Anne Overton
Director of Communications|
jao@bioaffinitytech.com
Investor Relations
Dave Gentry
RedChip Companies Inc.
1-800-RED-CHIP (733-2447)
Or 407-491-4498
BIAF@redchip.com
bioAffinity Technologies, Inc.
Condensed Consolidated Balance Sheets
| March 31, 2025 | December 31, 2024 | |||||||
| (unaudited) | ||||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 444,706 | $ | 1,105,291 | ||||
| Accounts and other receivables, net | 963,744 | 1,139,204 | ||||||
| Inventory | 38,782 | 27,608 | ||||||
| Prepaid expenses and other current assets | 416,550 | 422,995 | ||||||
| Total current assets | 1,863,782 | 2,695,098 | ||||||
| Non-current assets: | ||||||||
| Property and equipment, net | 382,409 | 375,385 | ||||||
| Operating lease right-of-use asset, net | 431,746 | 463,011 | ||||||
| Finance lease right-of-use asset, net | 684,629 | 780,872 | ||||||
| Goodwill | 1,404,486 | 1,404,486 | ||||||
| Intangible assets, net | 760,556 | 775,139 | ||||||
| Other assets | 19,675 | 19,676 | ||||||
| Total assets | $ | 5,547,283 | $ | 6,513,667 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 1,381,578 | $ | 987,311 | ||||
| Accrued expenses | 1,370,735 | 1,398,722 | ||||||
| Unearned revenue | 24,404 | 24,404 | ||||||
| Operating lease liability, current portion | 130,342 | 127,498 | ||||||
| Finance lease liability, current portion | 403,584 | 395,301 | ||||||
| Notes payable, current portion | 104,766 | 171,669 | ||||||
| Total current liabilities | 3,415,409 | 3,104,905 | ||||||
| Non-current liabilities | ||||||||
| Operating lease liability, net of current portion | 308,415 | 342,098 | ||||||
| Finance lease liability, net of current portion | 335,899 | 444,448 | ||||||
| Notes payable, net of current portion | 48,156 | 20,180 | ||||||
| Total liabilities | 4,107,879 | 3,911,631 | ||||||
| Commitments and contingencies (See Note 11) | ||||||||
| Stockholders’ equity: | ||||||||
| Preferred stock, no shares issued or outstanding at March 31, 2025, and December 31, 2024, respectively | — | — | ||||||
| Common stock, par value $0.007 per share; 100,000,000 shares authorized; 18,255,825 and 15,576,674 shares issued and outstanding as of March 31, 2025, and December 31, 2024, respectively | 124,777 | 106,593 | ||||||
| Additional paid-in capital | 57,619,354 | 56,139,753 | ||||||
| Accumulated deficit | (56,304,727 | ) | (53,644,310 | ) | ||||
| Total stockholders’ equity | 1,439,404 | 2,602,036 | ||||||
| Total liabilities, and stockholders’ equity | $ | 5,547,283 | $ | 6,513,667 | ||||
bioAffinity Technologies, Inc.
Unaudited Condensed Consolidated Statements of Operations
| Three Months Ended March 31, |
||||||||
| 2025 | 2024 | |||||||
| Net Revenue | $ | 1,853,597 | $ | 2,406,391 | ||||
| Operating expenses: | ||||||||
| Direct costs and expenses | 1,367,860 | 1,573,441 | ||||||
| Research and development | 367,386 | 393,639 | ||||||
| Clinical development | 138,353 | 48,960 | ||||||
| Selling, general and administrative | 2,452,549 | 2,185,944 | ||||||
| Depreciation and amortization | 154,588 | 149,637 | ||||||
| Total operating expenses | 4,480,736 | 4,351,621 | ||||||
| Loss from operations | (2,627,139 | ) | (1,945,230 | ) | ||||
| Other income (expense): | ||||||||
| Interest income | 542 | 6,127 | ||||||
| Interest expense | (15,485 | ) | (23,550 | ) | ||||
| Other income | 2 | 4,510 | ||||||
| Other expense | (9,642 | ) | — | |||||
| Total other expense | (24,583 | ) | (12,913 | ) | ||||
| Net loss before provision for income taxes | (2,651,722 | ) | (1,958,143 | ) | ||||
| Income tax expense | (8,695 | ) | (3,672 | ) | ||||
| Net loss | $ | (2,660,417 | ) | $ | (1,961,815 | ) | ||
| Net loss per common share, basic and diluted | $ | (0.16 | ) | $ | (0.20 | ) | ||
| Weighted average common shares outstanding, basic and diluted | 16,257,456 | 9,915,426 | ||||||
# # #