“Automated Flow Cytometry Test Distinguishes Cancer from Non-Cancer in Sputum with High Sensitivity and Specificity”
International Association for the Study of Lung Cancer (IASLC) 2020 World Conference on Lung Cancer, Jan. 2021
Authors
Lydia H. Bederka1, Patricia Araujo, Jamila Sanchez, Marcia Grayson, Madeleine E. Lemieux, Jennifer Rebeles, Shao-Chiang Lai, Xavier T. Reveles and Vivienne I. Rebel (presenter)
Summary
“Automated Flow Cytometry Test Distinguishes Cancer from Non-Cancer in Sputum with High Sensitivity and Specificity” poster presented at the International Association for the Study of Lung Cancer (IASLC) 2020 World Conference on Lung Cancer hosted by the International Association for the Study of Lung Cancer, Singapore, Worldwide Virtual Event (WCLC 2020), January 28- 31, 2021.
Introduction
Low-dose spiral computed tomography (LDCT) screening for lung cancer reduces mortality. It is therefore recommended in the U.S.A. for high-risk individuals between 55 and 80 years of age, who have smoked 30 pack years or more and have not quit smoking for more than 15 years. LDCT results may not always lead to a clear follow-up procedure when the nodules are small, particularly when they are between 4 mm to 20 mm in size. We therefore sought to develop a non-invasive test to detect lung cancer in individuals at high risk with emphasis on the test’s ability to distinguish cancer in individuals with smaller nodules.
Methods
We evaluated the ability of the CyPath® Lung flow cytometric test to correctly classify cohorts of high-risk controls (n = 122) and cancer (n = 28) subjects. Sputum was collected by participants at home over three days and shipped overnight to the laboratory. Sputum was processed upon receipt into a single-cell suspension before labeling with a viability dye to exclude dead cells, antibodies to distinguish cell types and a porphyrin label to identify cancer and/or cancer-associated cells. An automated flow cytometry data analysis pipeline was developed to capture potentially informative variables to distinguish cancer from non-cancer. Flow cytometric and patient features were evaluated individually and in combination as predictors of disease state. Subsets of samples (2/3) were randomly selected for training a generalized linear model, maintaining the relative proportion of cancer to high-risk samples in the whole group. The training set was used to fit the model while the remaining samples were used for independent validation. This process of random selection of training and test sets was repeated to identify a minimal set of predictive variables to avoid over-fitting of the model to the data. Further randomization was then used to evaluate the robustness of the resulting model.
Results
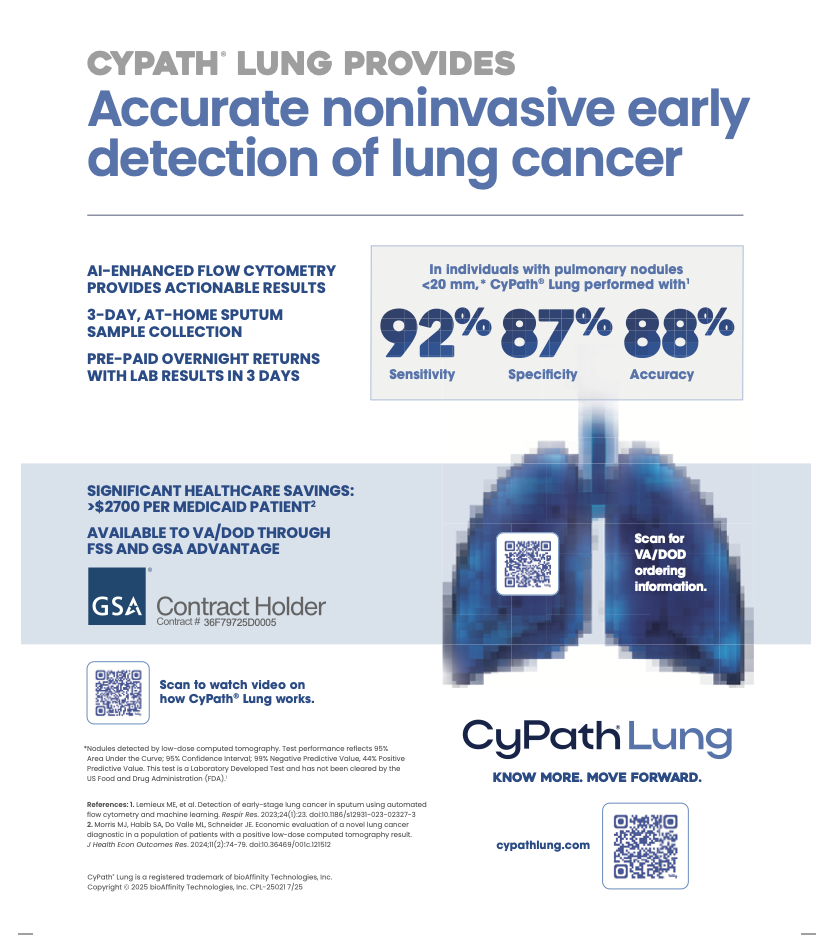
Automated analysis of sample data proved to be fast and identified four predictive parameters including a porphyrin label that had been shown to detect cancer in sputum in an earlier, slide-based version of the CyPath® Lung test (Patriquin et al, J Thorac Oncol. 2015). The model is robust in predicting cancer and high risk, with 88% specificity and 82% sensitivity on this population, a positive predictive value of 62% and a negative predictive value of 95%. The test was 92% sensitive and 87% specific in correctly classifying disease state in participants who had no nodules or nodules of less than 20 mm in diameter (104/119 high-risk control patients and 12/13 cancer patients). All high-risk controls with nodules larger than 20 mm in diameter (3 participants) and 75% of the cancer patients with nodules larger than 20 mm (9/12 participants) were correctly classified.
Conclusion
The automated CyPath® Lung flow cytometric assay correctly classifies study participants into cancer or high-risk cohorts with high accuracy, including participants with nodules smaller than 20 mm. The CyPath® Lung assay thus has the potential to complement LDCT screening and improve diagnosis of early stage lung cancer.
Access Full Paper Here
https://www.jto.org/article/S1556-0864(21)00459-7/