As published by 360Dx, March 14, 2025
By Greg Cima
NEW YORK – BioAffinity Technologies said that it is cutting costs in its pathology lab and preparing to start a clinical trial for its sputum-based lung cancer test in a bid to expand into a national player in cancer diagnostics.
Maria Zannes, BioAffinity Technologies president and CEO, said that the San Antonio, Texas-based company is poised to begin recruitment of upward of 3,200 patients for a trial to show the efficacy of its CyPath Lung test and support a submission for US Food and Drug Administration marketing clearance, likely through the agency’s de novo pathway. The firm currently offers the test as a laboratory-developed test (LDT) and performed about 620 tests in 2024, primarily in Texas.
Its efforts to expand the reach of the test comes as the firm faces possible delisting action by the Nasdaq. The company said last month in a filing with the US Securities and Exchange Commission that it had received notice from the Nasdaq that its common stock bid price had closed below the required minimum of $1.00 per share for 30 consecutive business days. BioAffinity reported that it is monitoring its stock price and considering its options to regain compliance by August 6, after which the company may be eligible for additional time to comply with the rule.
At the close of the market on Thursday, shares of BioAffinity Technologies were priced at $.35.
Nonetheless, BioAffinity is moving on with its plans. In the fall of 2023, the firm created its Precision Pathology Laboratory Services (PPLS) subsidiary through the $3.5 million acquisition of Village Oaks Pathology services, which had operated under the name Precision Pathology Services. The lab had licensed BioAffinity’s technologies and developed CyPath Lung as an LDT, and BioAffinity said at the time that the deal provided full control over the development and marketing of the assay as it laid the groundwork for national expansion.
BioAffinity announced earlier this month that it is cutting $4 million in annual costs by laying off 38 percent of PPLS staff, cutting services and supplies spending, and cutting certain pathology services with suboptimal margins. The firm declined to say how many employees were affected by the cuts or remained at the lab, although it said in 2023 that the post-acquisition firm would have 72 employees.
Zannes added that the many of the cuts are occurring in the company’s sexually transmitted infection testing services.
“We needed to make sure that all of our operations with Precision support CyPath lung and also support a good profit margin,” she said.
CyPath Lung is used for the early detection of lung cancer in patients who have suspicious CT results. Patient-collected sputum samples are treated with the fluorescent porphyrin compound meso-tetra (4-carboxyphenyl) porphine, or TCPP, which binds to cancer cells and cancer-related cells, as well as fluorescently labeled antibodies that are used for the identification of hematopoietic and epithelial cells. Flow cytometry and an AI-developed algorithm are used for the identification of malignancies.
Zannes said that the test is typically performed on samples from patients who have indeterminate pulmonary nodules, or growths that may be cancerous. The test can be used to help determine the next steps for a patient, including navigational bronchoscopy, fine-needle biopsy, or surgical biopsy.
She said, however, that clinicians have also reported using the test when they were skeptical of negative results from navigational bronchoscopy, with at least a few of the test results helping to identify cancers.
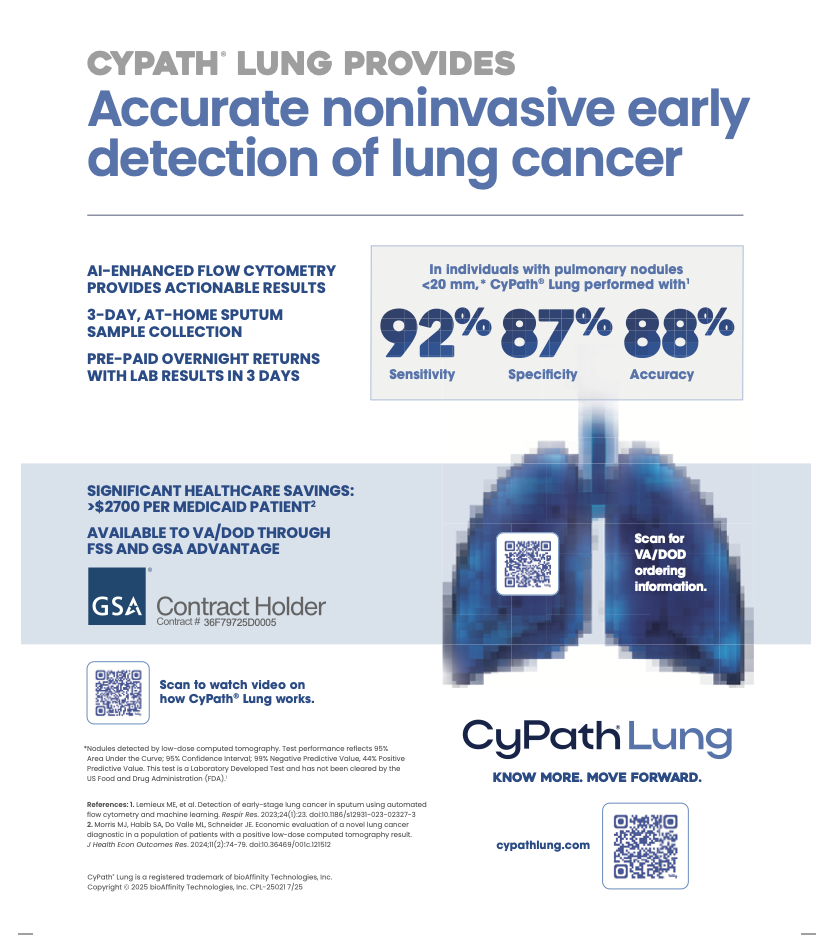
BioAffinity reported in 2023 in Respiratory Research that the test was used in a 150-patient validation trial, and it performed with 82 percent sensitivity and 88 percent specificity among patients with increased risk of lung cancer. The researchers wrote that the test had higher performance in patients with lung nodules of 20 mm or less, with 92 percent sensitivity and 87 percent specificity. Among the patients, 28 had confirmed diagnoses of lung cancer.
The company received in 2023 a current procedural terminology (CPT) code for CyPath Lung and a determination from the US Centers for Medicare and Medicaid Services that the test would be added to the 2024 clinical laboratory fee schedule at $1,900 per test. In October 2024, the company announced that CyPath Lung had been added to the US Federal Supply Schedule procurement system for the Veterans Health Administration within the US Department of Veterans Affairs and the US Department of Defense’s Military Health System. The firm noted that the VA promotes annual lung cancer screening for high-risk patients and the inclusion of the company’s test in the procurement system could provide a revenue stream.
Researchers from the DoD, VA, and Avalon Health Economics reported last year in the Journal of Health Economics and Outcomes Research that the use of CyPath Lung in patients at elevated risk of lung cancer due to indeterminate pulmonary nodules could reduce costs compared to typical expenses including imaging-based follow-up assessments, biopsies, and complications from those procedures. The authors reported that the use of CyPath lung could reduce costs by $2,733 per patient with Medicare coverage and $6,460 per patient with private insurance coverage.
Zannes said that the growing coverage CyPath Lung among government and private payors, its addition to the federal procurement system, and the positive results from the economic analysis have together given BioAffinity an array of tools that it can use when talking with physicians about the test. The company began its expansion locally in Texas and has been growing its sales force since last year.
“We look, this year, to start to sell into the VA and…bring those cost savings to the VA,” she said.
The company also announced last October that the Japan Patent Office had granted a patent for CyPath Lung as a test for lung cancer, bringing the number of patents BioAffinity holds globally to 17 in addition to its 30 pending patent applications.
The market for early cancer detection tests continues to heat up, with up-and-coming tests for indications such as pancreatic and breast cancers. Exact Sciences plans to soon offer a multi-cancer early cancer detection and screening test, while sales of Grail’s Galleri test are increasing. In the lung cancer testing space, Oxford Cancer Analytics recently said that it will use $11 million in Series A financing to develop and commercialize its protein-based assay for lung cancer screening.
As for BioAffinity’s upcoming clinical trial, Zannes said that it bolstered its plans following consultation with the FDA. She also noted that officials at more than 20 VA facilities have expressed interest in participating in the trial, and BioAfffinity plans to seek participation from VA facilities that have large-scale lung cancer screening programs along with large academic institutions.
The trial will involve the recruitment of patients with lung nodules of 6-20 mm and testing those patients for up to two years to reach a definitive diagnosis, she said. The results also could provide some early evidence how the test performs over time and whether it could be used as a screening test, although she said that a larger trial would be needed to support that indication.
According to the most recently available financial information filed with the SEC in November, BioAffinity recorded revenues of $7.2 million for the first nine months of 2024 compared to $319,000 in the first nine months of 2023. However, the firm still posted a loss of $6.0 million during that period, up from $5.6 million a year earlier, due to rising costs and increased SG&A spending.
BioAffinity also said in March 2024 that it had raised $2.5 million in gross proceeds through a registered direct offering and concurrent private placement, and it said in October that it had raised another $2.7 million through a registered direct offering and concurrent private placement. In both instances, the company said that the money would be used for working capital and general corporate purposes.