bioAffinity Technologies Reports $2.4 Million Revenue for Q2 Driven by Growing CyPath® Lung Sales
Q2 CyPath® Lung test sales up 217% over Q1 2024
Expanded CyPath® Lung test sales to physicians in California and Ohio; now receiving orders from physicians in eight states outside of Texas
Raised full-year CyPath® Lung sales forecast for test marketing program by 85% during the second quarter
SAN ANTONIO, TX (August 14, 2024) – bioAffinity Technologies, Inc. (Nasdaq: BIAF; BIAFW), a biotechnology company focused on the need for noninvasive, accurate tests for the detection of early-stage lung cancer and other lung diseases, today reported financial results for the three months ended June 30, 2024.
Key Highlights
- Generated quarterly revenue of $2.4 million in the second quarter of 2024.
- Limited market launch in Texas has realized more than 963% annualized growth rate for CyPath® Lung orders in first six months of 2024 over full-year 2023, with second quarter 2024 sales up 217% over first quarter 2024 sales.
- Forecasting upwards of $9.6 million in 2024 revenues for wholly owned subsidiary Precision Pathology Laboratory Services (PPLS), up 23% over 2023.
- Increased 2024 CyPath® Lung sales forecast 85% from the original forecast reported in the 2023 Annual Report, which would result in a 2,429% year-over-year full-year growth.
- Number of physician offices ordering CyPath® Lung is up 144% since Jan. 1, 2024.
- Referrals and word-of-mouth from physicians, including key opinion leaders (KOLs), continue to drive strong uptake of CyPath® Lung in states beyond Texas; now receiving CyPath® Lung orders from physicians in eight states, up from six in the first quarter of 2024. In addition to previously reported orders from Pennsylvania, New Jersey, North Carolina, Arizona, and Michigan, physicians in California and Ohio have also begun ordering CyPath® Lung tests.
- Continued to advance new product development initiatives in collaboration with the U.S Department of Defense’s largest military health organization, focusing on tests that use our artificial intelligence and flow cytometry platform for diagnosing COPD and companion test with bronchoscopy.
- Successfully closed a $1.75 million registered direct offering and concurrent private placement to fund continued growth.
Management Commentary
“We are encouraged by the momentum we’ve achieved in the second quarter, with CyPath® Lung sales growing 217% over the previous quarter and exceeding our initial forecasts,” bioAffinity President and Chief Executive Officer Maria Zannes said. “This growth underscores the increasing recognition of the role CyPath® Lung can play in the early detection of lung cancer, a disease where early diagnosis can significantly improve patient outcomes. As we expand our reach beyond Texas, the positive reception from physicians across multiple states reinforces our belief that CyPath® Lung will have a significant impact in lung cancer diagnostics, a U.S. market projected to reach $4.7 billion by 2030.”
“Our strategic focus remains on scaling our operations and solidifying our position in this market, supported by a strong foundation of word-of-mouth referrals and physician enthusiasm,” Zannes continued. “With our experienced sales and support teams in place, we are well-prepared to meet the growing demand and continue market expansion. As we look ahead, we believe our efforts will not only drive further growth but also bring us closer to our mission of improving patient lives.”
Second Quarter Financial Results
Revenue for the second quarter of 2024 was $2.4 million, compared with $20,000 revenue for the prior-year period. The majority of the year-over-year increase is through the acquisition of PPLS. Revenue is primarily generated from patient service fees, including billing for CyPath® Lung tests, with additional revenues generated from histology service fees and medical director fees.
Research and development expenses were $402,000 for the second quarter of 2024, compared with $335,000 for the comparable period in 2023. The increase was primarily due to higher compensation costs for additional research personnel and higher R&D laboratory supply costs.
Clinical development expenses were $51,000 for the second quarter of 2024, compared with $35,000 for the second quarter of 2023. The increase was primarily attributable to an increase in compensation costs and benefits from the addition of new clinical development personnel.
Selling, general and administrative expenses were $2.5 million for the second quarter of 2024, compared with $1.4 million for the comparable period in 2023. The increase was primarily attributed to acquired general and administrative costs from PPLS and an increase in personnel and services to support the launch of CyPath® Lung.
Net loss for the second quarter of 2024 was $2.1 million, or $0.19 per share, compared with a net loss of $1.7 million, or $0.20 per share, for the comparable period in 2023.
Cash and cash equivalents as of June 30, 2024, were $0.8 million, compared with $2.8 million as of December 31, 2023. bioAffinity Technologies raised aggregate gross proceeds of $1.75 million in a registered direct offering, concurrent private placement, and warrant inducement transaction that closed on Aug. 5, 2024.
About CyPath® Lung
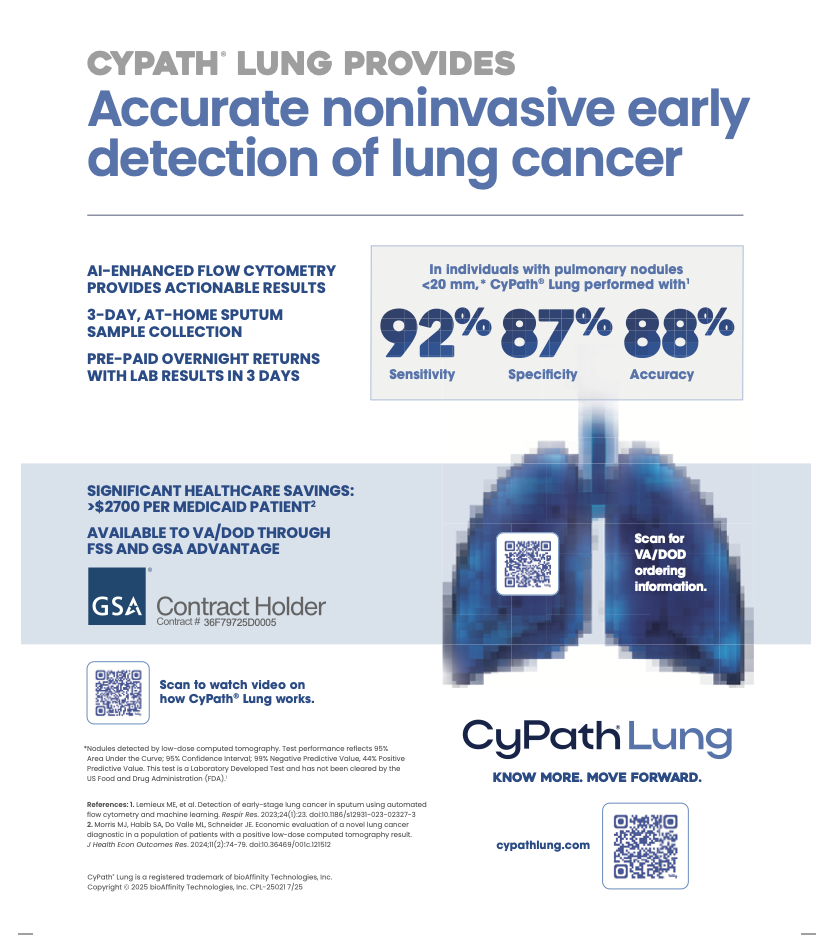
CyPath® Lung uses proprietary advanced flow cytometry and artificial intelligence (AI) to identify cell populations in patient sputum that indicate malignancy. Automated data analysis helps determine if cancer is present or if the patient is cancer-free. CyPath® Lung incorporates a fluorescent porphyrin that is preferentially taken up by cancer and cancer-related cells. Clinical study results demonstrated that CyPath® Lung had 92% sensitivity, 87% specificity and 88% accuracy in detecting lung cancer in patients at high risk for the disease who had small lung nodules less than 20 millimeters. Diagnosing and treating early-stage lung cancer can improve outcomes and increase patient survival. For more information, visit www.cypathlung.com.
About bioAffinity Technologies, Inc.
bioAffinity Technologies, Inc. addresses the need for noninvasive diagnosis of early-stage cancer and diseases of the lung and broad-spectrum cancer treatments. The Company’s first product, CyPath® Lung, is a noninvasive test that has shown high sensitivity, specificity and accuracy for the detection of early-stage lung cancer. CyPath® is marketed as a Laboratory Developed Test (LDT) by Precision Pathology Laboratory Services, a subsidiary of bioAffinity Technologies. For more information, visit www.bioaffinitytech.com.
Forward-Looking Statements
Certain statements in this press release constitute “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “might,” “will,” “should,” “believe,” “expect,” “anticipate,” “estimate,” “continue,” “predict,” “forecast,” “project,” “plan,” “intend” or similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. These forward-looking statements are based upon current estimates and assumptions and include statements regarding raising full-year CyPath® Lung sales forecast for test marketing program by 85% during the second quarter which would result in a 2,429% year-over-year full-year growth, forecasting upwards of $9.6 million in 2024 revenues for Precision Pathology Laboratory Services, up 23% over 2023, referrals and word-of-mouth from physicians, including KOLs, continuing to drive strong uptake of CyPath® Lung in states beyond Texas, advancing new product development initiatives in collaboration with the U.S Department of Defense’s largest military health organization, focusing on tests that use the Company’s artificial intelligence and flow cytometry platform for diagnosing COPD and companion test with bronchoscopy, the increasing recognition of CyPath® Lung’s critical role in the early detection of lung cancer, CyPath® Lung setting a new standard in lung cancer diagnostics, scaling the Company’s operations and solidifying its position in the lung cancer diagnostics market, being well-prepared with the Company’s enhanced sales and support teams in place to meet the growing demand and continue its national expansion, and the Company’s efforts to drive further growth and bring it closer to its mission of improving patient lives through innovative, noninvasive cancer diagnostics. These forward-looking statements are subject to various risks and uncertainties, many of which are difficult to predict that could cause actual results to differ materially from current expectations and assumptions from those set forth or implied by any forward-looking statements. Important factors that could cause actual results to differ materially from current expectations include, among others, the Company’s ability to continue to accelerate the commercialization of CyPath® Lung and capitalize on the lung cancer diagnostics market; the ability of CyPath® Lung to provide the anticipated benefits to patients and physicians; and the other factors discussed in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, and its subsequent filings with the SEC, including subsequent periodic reports on Forms 10-Q and 8-K. Such forward-looking statements are based on facts and conditions as they exist at the time such statements are made and predictions as to future facts and conditions. While the Company believes these forward-looking statements are reasonable, readers of this press release are cautioned not to place undue reliance on any forward-looking statements. The information in this release is provided only as of the date of this release, and the Company does not undertake any obligation to update any forward-looking statement relating to matters discussed in this press release, except as may be required by applicable securities laws.
Contacts
bioAffinity Technologies
Julie Anne Overton
Director of Communications
jao@bioaffinitytech.com
Investor Relations
Dave Gentry
RedChip Companies Inc.
1-800-RED-CHIP (733-2447) Or 407-491-4498
BIAF@redchip.com
bioAffinity Technologies, Inc.
Condensed Consolidated Balance Sheets
| June 30, 2024 | December 31, 2023 | |||||||
| (unaudited) | ||||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 801,311 | $ | 2,821,570 | ||||
| Accounts and other receivables, net | 1,595,626 | 811,674 | ||||||
| Inventory | 29,768 | 18,484 | ||||||
| Prepaid expenses and other current assets | 253,726 | 321,017 | ||||||
| Total current assets | 2,680,431 | 3,972,745 | ||||||
| Non-current assets: | ||||||||
| Property and equipment, net | 449,250 | 458,633 | ||||||
| Operating lease right-of-use asset, net | 324,942 | 370,312 | ||||||
| Finance lease right-of-use asset, net | 973,358 | 1,165,844 | ||||||
| Goodwill | 1,404,486 | 1,404,486 | ||||||
| Intangible assets, net | 804,306 | 833,472 | ||||||
| Other assets | 19,675 | 16,060 | ||||||
| Total assets | $ | 6,656,448 | $ | 8,221,552 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 848,102 | $ | 604,789 | ||||
| Accrued expenses | 969,093 | 1,149,811 | ||||||
| Unearned revenue | 26,135 | 33,058 | ||||||
| Operating lease liability, current portion | 98,593 | 94,708 | ||||||
| Finance lease liability, current portion | 380,259 | 365,463 | ||||||
| Notes payable, current portion | 4,106 | — | ||||||
| Total current liabilities | 2,326,288 | 2,247,829 | ||||||
| Non-current liabilities: | ||||||||
| Finance lease liability, net of current portion | 641,566 | 835,467 | ||||||
| Operating lease liability, net of current portion | 232,714 | 283,001 | ||||||
| Notes payable, net of current portion | 22,766 | — | ||||||
| Total liabilities | 3,223,334 | 3,366,297 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ equity: | ||||||||
| Preferred stock, par value $0.001 per share; 20,000,000 shares authorized; no shares issued or outstanding at June 30, 2024, and December 31, 2023 | — | — | ||||||
| Common stock, par value $0.007 per share; 100,000,000 shares authorized; 11,487,046 and 9,394,610 issued and outstanding at June 30, 2024, and December 31, 2023, respectively | 79,407 | 65,762 | ||||||
| Additional paid-in capital | 52,030,280 | 49,393,972 | ||||||
| Accumulated deficit | (48,676,573 | ) | (44,604,479 | ) | ||||
| Total stockholders’ equity | 3,433,114 | 4,855,255 | ||||||
| Total liabilities and stockholders’ equity | $ | 6,656,448 | $ | 8,221,552 | ||||
bioAffinity Technologies, Inc.
Unaudited Condensed Consolidated Statements of Operations
| Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||||
| 2024 | 2023 | 2024 | 2023 | |||||||||||||
| Net Revenue | $ | 2,397,652 | $ | 19,738 | $ | 4,804,043 | $ | 20,659 | ||||||||
| Operating expenses: | ||||||||||||||||
| Direct costs and expenses | 1,407,710 | 1,234 | 2,981,151 | 1,322 | ||||||||||||
| Research and development | 402,433 | 335,125 | 796,072 | 704,741 | ||||||||||||
| Clinical development | 51,462 | 35,260 | 100,422 | 54,888 | ||||||||||||
| Selling, general, and administrative | 2,472,775 | 1,404,917 | 4,658,719 | 2,552,792 | ||||||||||||
| Depreciation and amortization | 151,070 | 21,552 | 300,707 | 43,236 | ||||||||||||
| Total operating expenses | 4,485,450 | 1,798,088 | 8,837,071 | 3,356,979 | ||||||||||||
| Loss from operations | (2,087,798 | ) | (1,778,350 | ) | (4,033,028 | ) | (3,336,320 | ) | ||||||||
| Other income (expense): | ||||||||||||||||
| Interest income | 5,186 | 44,124 | 11,313 | 82,778 | ||||||||||||
| Interest expense | (22,249 | ) | (1,360 | ) | (45,799 | ) | (3,015 | ) | ||||||||
| Other expense | 1 | — | 4,511 | — | ||||||||||||
| Total other income (expense) | (17,062 | ) | 42,764 | (29,975 | ) | 79,763 | ||||||||||
| Net loss before provision for income tax expense | (2,104,860 | ) | (1,735,586 | ) | (4,063,003 | ) | (3,256,557 | ) | ||||||||
| Income tax expense | 5,419 | 4,587 | 9,091 | 16,406 | ||||||||||||
| Net loss | $ | (2,110,279 | ) | $ | (1,740,173 | ) | $ | (4,072,094 | ) | $ | (3,272,963 | ) | ||||
| Net loss per common share, basic and diluted | $ | (0.19 | ) | $ | (0.20 | ) | $ | (0.38 | ) | $ | (0.38 | ) | ||||
| Weighted average common shares outstanding | 11,389,308 | 8,520,714 | 10,655,483 | 8,477,656 | ||||||||||||
# # #