Local CEO: New recommendations acknowledge that women and Black people are at higher risk than white men
MARCH 19, 2021
As published in the San Antonio Business Journal
By: W. Scott Bailey – Senior Reporter, San-Antonio Business Journal
Several major national medical groups are calling on the Centers for Medicare & Medicaid Services to update lung cancer screening coverage to significantly expanding access to such tests.
And if they convince CMS to follow new U.S. Preventive Services Task Force recommendations, one San Antonio biotech could see its business grow exponentially.
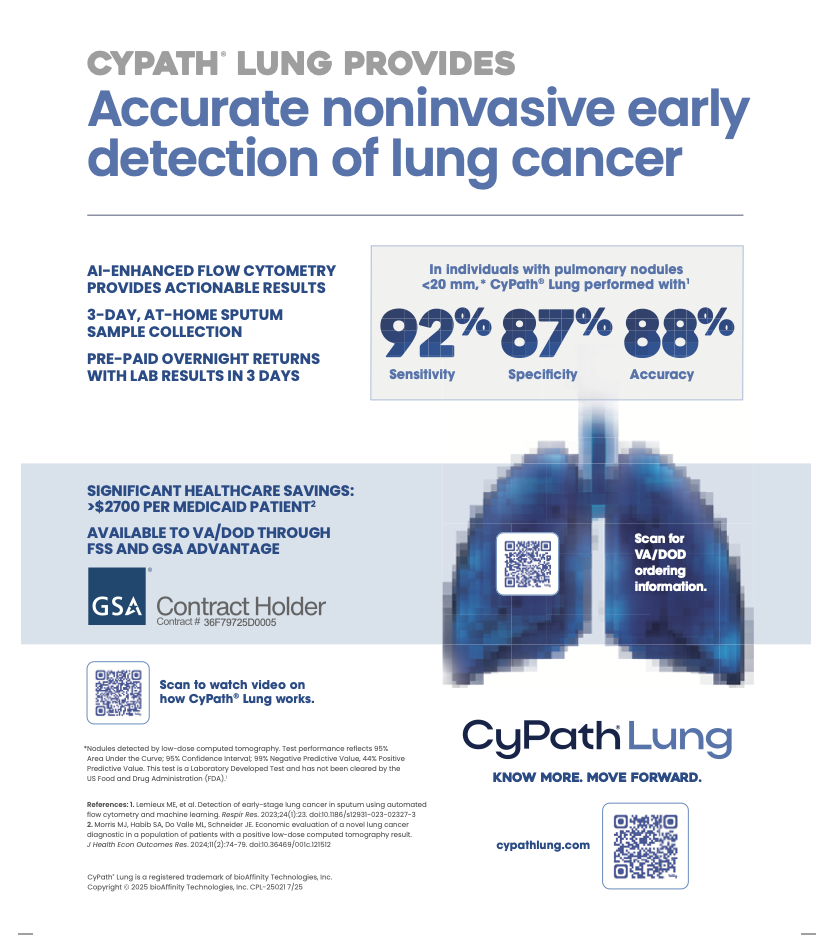
BioAffinity Technologies Inc., which is developing tests and therapies to detect and treat cancer at the cellular level, is advancing commercialization of its initial product — CyPath® Lung, a noninvasive test for early detection of lung cancer.
“The [Preventive Services Task Force] recommendations nearly double the U.S. market for CyPath® Lung,” bioAffinity CEO Maria Zannes said. “Most important, many more lives will be saved by catching lung cancer early — when it can be treated.
The American College of Radiology, the GO2 Foundation for Lung Cancer and The Society of Thoracic Surgeons have urged CMS to open a National Coverage Determination reconsideration on lung cancer screening.
The groups are specifically calling on CMS to lower the age for screening eligibility to 50 and to eliminate the requirement that individuals receiving the screenings be current smokers or people who have quit smoking in the last 15 years.
“We had been expecting these recommendations for some time, and we are very pleased to see them issued,” Zannes said. “It is particularly important that the U.S. Preventative Service Task Force expanded eligibility for screening to acknowledge that women and the Black community have a higher risk of lung cancer at an earlier age and with a lesser smoking history than white males, on which the current recommendations are based.”
If the recommendations are adopted, Zannes said it would nearly double the number of people who would be recommended for lung cancer screenings.
BioAffinity officials note that CyPath® Lung has proven to be highly accurate in finding early-stage cancer in people at high risk for the disease, whereas low-dose CT screenings can produce false positives, leading to potentially costly and invasive follow-up tests and even biopsies.