With a San Antonio biotech firm’s test, your phlegm could help save you from cancer.
As published by the San Antonio Express-News
By Brandon Lingle, Staff Writer
The screening method developed by bio Affinity Technologies Inc. uses Al–aided computer analysis of a patient’s phlegm in a test that can spot lung cancer in high–risk patients earlier than other diagnosis methods. That can be the difference between starting treatment when it’s needed or waiting until it may be too late.
“So many times what a physician will say is, ‘Let’s wait – you have a nodule, it’s concerning, come back in six months.‘ Or come back in eight months, and you can imagine for people that can cause a lot of anxiety,” said CEO Maria Zannes. “That’s where our test comes in … where there is some kind of question as to whether someone has lung cancer (and) they don’t want to go in and do a biopsy right away.”
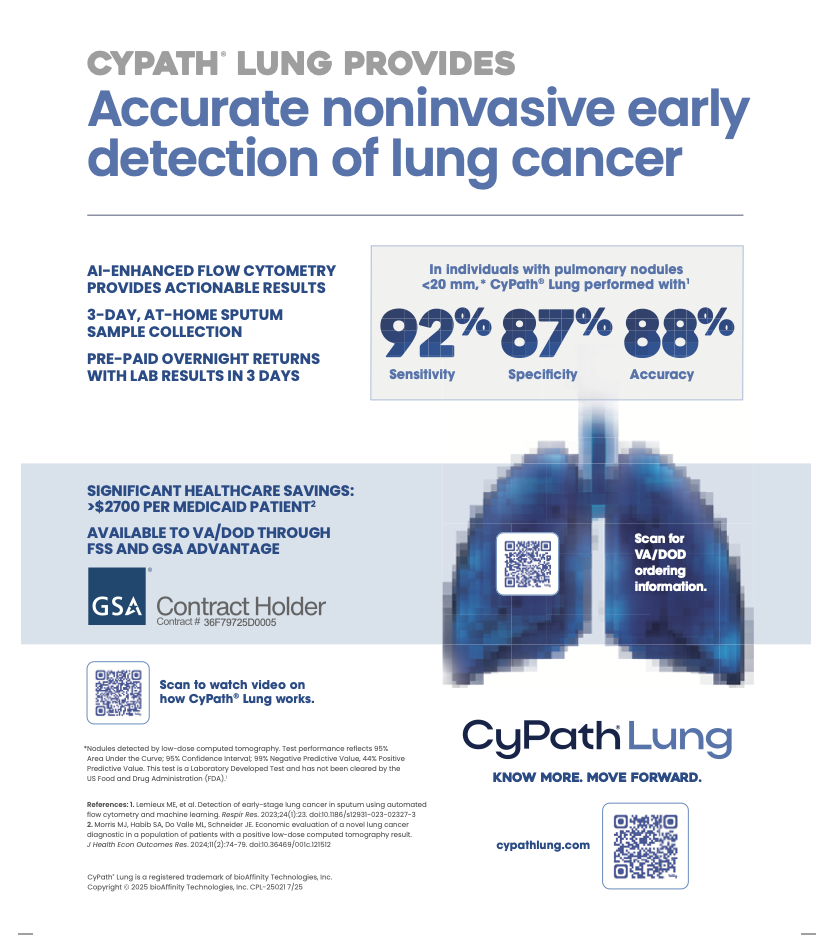
Since being launched last year, bioAffinity’s CyPath Lung test has been fueling rapid growth for the company as its market expands across the U.S. That pace is likely to increase after it was awarded a long–term federal contract Wednesday that will streamline the test’s availability to patients served by the Veterans Health Administration and Military Health System.
With CyPath Lung, bioAffinity is aiming to fill a gap in a large and growing market, Zannes said. Lung cancer kills more people worldwide than any other type of cancer, according to the World Health Organization. Doctors can treat it if caught early but early detection can be difficult with current testing methods.
The noninvasive CyPath test can help when symptoms or prior test results require more investigation, she said. It only requires the subject taking a shower, breathing into a special device, spitting into a cup and mailing in the sample. Other current testing methods include CT scans and biopsies.
Rapid growth
Since the test received federal approval for patient use last year, it’s seen rapid acceptance by doctors and skyrocketing growth in orders. Interest in the $1,900 tests picked up after insurance, including Medicare, began reimbursing the costs.
In the three months through June, total company sales were up 217% from the year’s first three months. Second–quarter revenue was $2.4 million, up from less than $20,000 a year earlier. Since Jan. 1, the number of physician offices ordering the test is up 144%.
“We’re projecting about 800 tests this year and then moving on from there,” Zannes said before the federal contract was announced.
The contract announced Wednesday is likely to boost those projections. Through the Veterans Health Administration alone, about 8,000 veterans are diagnosed and treated for lung cancer annually, according to the Department of Veterans Affairs.
The company was launched in 2014 and has been working since to perfect and bring its CyPath Lung to market. It’s been publicly traded since an initial public offering in 2022, a move Zannes said has helped the company grow.
“It’s been great to be in the public markets, and we’ve been able to access funds,” she said. “It is a difficult time for all small cap companies, but we’ve done okay.”
As of Friday, the company’s market capitalization was $26.14 million.
It has about 100 employees working at its lab and headquarters on the 3300 block of Nacogdoches Road and a research facility on the University of Texas at San Antonio campus.
After rolling out its flagship test first in Texas and across the Southwest, bioAffinity is expanding it to other markets in the U.S. and planning to take it to Europe and, eventually, Southeast Asia.
In the U.S., the test’s acceptance has been aided by studies that found it both effective and economical.
A 2023 study published in the journal Respiratory Research concluded the test classified samples as cancer or non–cancer with “high accuracy.” And in mid-September, another study published in the Journal of Health Economics and Outcomes Research found that the test could save each patient between about $3,000 and $6,000 in medical expenses by catching the disease early.
It found the cost savings are “primarily due to reductions in follow–up diagnostic assessments and procedures.” Among the study’s co–authors were Dr. Michael J. Morris, a Brooke Army Medical Center pulmonology and critical care physician, and Dr. Sheila A. Habib, director of the Pulmonary Lung Nodule Clinic at Audie L. Murphy Memorial Veterans Hospital.
The Test
Among the reasons for the test’s rapid acceptance is that it’s non–invasive and can be done by patients at home.
It begins with a shower, which helps break up mucus in the lungs. Then, the patient breathes into an Acapella airway clearing device, a handheld plastic tool that further helps dislodge mucus. Next, they spit into a cup. Three such samples are collected over three days before returning it to the lab.
Upon arriving at Precision Pathology Laboratory Services, a bioAffinity–owned subsidiary, technicians begin a two–day analysis process that involves flow cytometry, a way of measuring cell characteristics with lasers.
The technique has been around since the 1960s and is widely used to diagnose blood cancers. But Zannes said her company adapted the technology to analyze sputum and screen for lung cancer.
The “more groundbreaking” aspect, she said: “We’ve automated it.“
Using machine learning, a subset of artificial intelligence, the company developed an algorithm to analyze the cytometry data to determine the patient’s likelihood of having lung cancer.
“After the sample runs through the flow, which takes about half hour, the data goes into this algorithm and out comes the result,” she said.
It looks at cell populations and how the lung environment is changing.
While the mucus is inside the lungs it captures cells and other markers that can provide hints to the organ’s health. One such marker is a porphyrin, an organic compound, that marks cancer and cancer–related cells.
“There’s a battle going on in the lungs,” Zannes said. If cancer is there “you’re going to have more cancer cells, you’re going to have more dead cells and you’re going to have more immune cells, because the immune system is started to fight the cancer.“
Automation is important because each sample might have 16 million cells, and each cell has a dozen or more parameters to evaluate.
“It’s big, big data,” Zannes said, and “machine learning very quickly can determine what kind of (cell) populations are there.“
The system provides a report that says whether the patient is “very unlikely, unlikely, likely or very likely” to have a malignancy.
What’s Next
Looking forward, Zannes said the company wants to continue its expansion in the U.S. and beyond – and through the military long–term contract. It’s also continued to raise funds through private investment.
“There are certain areas where we’ve already seen significant interest – New Jersey, for example, we have several larger practices there that we’d like to talk to,” she said. “So, I think we’ll strategically grow the business.“
The military contract will play a part in that.
“Lung cancer is the leading cause of cancer–related death in veterans, despite being one of the most preventable cancers in the world,” Zannes said in a statement. “My father was one such veteran who died from lung cancer at age 39. That is just one of many reasons I am immensely proud that VA and Department of Defense physicians will be able to order CyPath Lung for their patients to help detect early–stage lung cancer with the goal of leading to better treatment and longer lives.“
The other part of bio Affinity’s strategy growth is bringing other tests to market. The company now is looking to apply the same technology to screen for chronic obstructive pulmonary disease.
“Finding COPD early has a similar positive effect on people,” Zannes said. “It can slow the disease and people can start to make changes that can make a difference and give them a longer life.”