As published in the San Antonio Business Journal, March 7, 2025
By W. Scott Bailey – Senior Reporter
BioAffinity Technologies Inc., one of San Antonio’s more promising early-stage biotech companies, is making tactical changes to improve its financial performance and accelerate commercial growth opportunities for its core product.
One of the bigger moves is to cut operating costs by $4 million, mostly through its subsidiary company, Precision Pathology Laboratory Services.
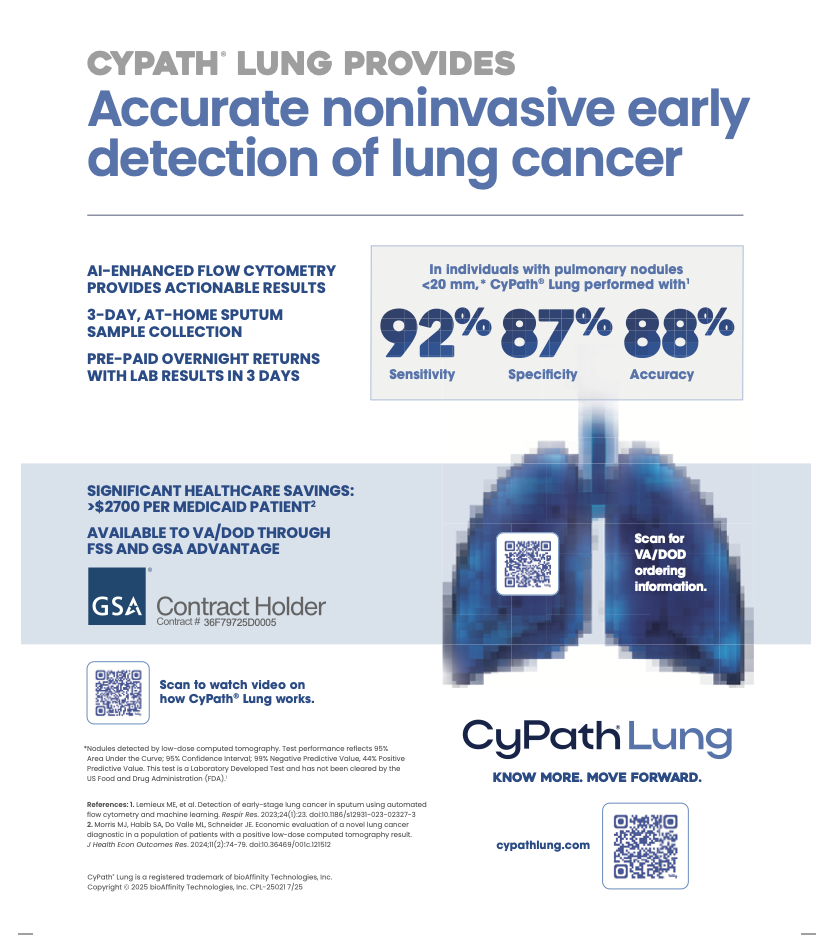
Simultaneously, bioAffinity will increase resources to expand sales of CyPath Lung, a noninvasive cancer detection technology, in what it’s deeming “high potential” national markets.
“We evaluate our entire business on a regular basis. In our most recent evaluation, we determined that we would no longer offer certain gynecological testing services based on suboptimal reimbursement,” bioAffinity founder and CEO Maria Zannes told me.
That pivot has reduced Precision Pathology’s workforce by nearly 40%. Zannes said that operational adjustment will allow bioAffinity and Precision Pathology to focus on new growth opportunities.
“This was a business decision,” Zannes said. “But it does not lessen the impact on people to whom we are grateful.”
Zannes said that bioAffinity, which has pursued growth opportunities domestically and internationally, will continue to rely on the combined innovation and expertise of the two companies to achieve certain critical milestones. Those include a 2025 enrollment for a pivotal U.S. Food and Drug Administration trial for CyPath Lung and the development of other noninvasive diagnostics, including tests for chronic obstructive pulmonary disease and asthma.
As part of its operational adjustment, bioAffinity will focus on high-margin services as it discontinues certain other operations. While the parent company anticipates these changes will decrease revenue, they are also expected to improve Precision Pathology’s overall profitability, according to bioAffinity Chief Financial Officer Michael Edwards.
CyPath Lung is now available for purchase through the Federal Supply Schedule. BioAffinity plans to launch a government-focused marketing program next quarter to introduce its test to the Veterans Health Administration and Department of Defense medical centers.
“Our strategic adjustments enhance our readiness to serve the market for noninvasive lung cancer detection in both the civilian and military healthcare systems,” said Zannes, who has remained steadfast in her efforts to grow the company.