The Alamo City biotech has secured a key win in Japan that could make it a key player in a nearly billion-dollar industry.
As published in the San Antonio Business Journal
By W. Scott Bailey – Senior Reporter, San Antonio Business Journal
BioAffinity Technologies Inc. has secured a Certificate of Grant of Patent from the Japan
Patent Office.
It’s a landmark win for one of San Antonio’s more promising biotech companies, opening the door for commercialization of its core product, CyPath Lung, in the Asian market.
The Japanese patent is an important addition to bioAffinity Technologies’ (NASDAQ: BIAF) patent portfolio. The Alamo City company currently has 17 U.S. and foreign patents, as well as 30 pending patent applications related to its noninvasive, early cancer detection diagnostic platform.
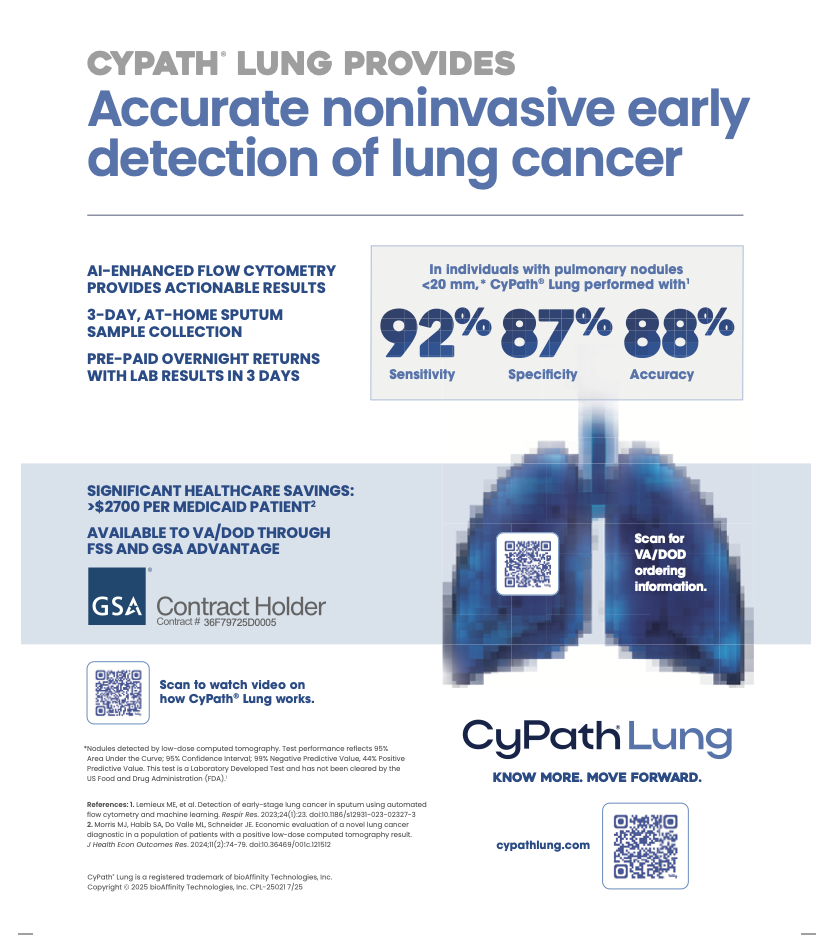
The Japanese patent is the first awarded for the CyPath Lung flow cytometry test as a stand-alone assay for the detection of lung cancer.
“Our Health of the Lung patent awarded in Japan is important for its confirmation that our novel and high performing test, CyPath Lung, can be protected on the global stage,” bioAffinity CEO Maria Zannes told me. “BioAffinity Technologies has a robust patent portfolio, including multiple patents that protect our noninvasive test for detecting early-stage lung cancer.”
The Japanese patent is the latest score for bioAffinity in its march to market. A few weeks ago, I reported that the company’s CyPath Lung test will be added to the U.S. Federal Supply Schedule, a procurement system that provides the Veterans Health Administration and Military Health System access to breakthrough health care products.
It’s a significant accomplishment for the roughly 10-year-old company and for a San Antonio bioscience industry that’s worked to align with military medicine. The VHA, part of the U.S. Department of Veterans Affairs, serves more than 9 million veterans annually and is the largest integrated health care system in the country, providing care at nearly 1,400 medical facilities.
The listing on the Federal Supply Schedule opens up a much larger, nationwide market for CyPath Lung, said Zannes, whose father, a veteran, died of lung cancer at the age of 39.
As bioAffinity pursues wider commercialization of its core product, it’s pursuing additional protection of its intellectual property.
“We have a number of patent applications that we expect to be awarded in the near future,” Zannes said.
Lung cancer is one of the deadliest cancers worldwide. BioAffinity officials believe the company has an opportunity to improve the outcome for lung cancer patients internationally through its CyPath Lung technology.
The company’s scientific team continues research and development work on other diagnostics that will open new markets to bioAffinity. Meanwhile, it has high expectations for the Asian market and the adoption of its core technology.
The Asian market for lung cancer diagnostics, including Japan, is estimated to grow into as much as a nearly $900 million industry by 2028, Zannes said.