“Sputum-Derived Cellular Profiles Produced by Flow Cytometric Analysis, Poster”
International Society for Advancement of Cytometry CYTO 2020, August 4-5, 2020
Authors
Lydia H. Bederka, Shao-Chiang Lai, Jennifer Rebeles, Marcia H. Grayson, Xavier T. Reveles, and Vivienne I. Rebel
Abstract
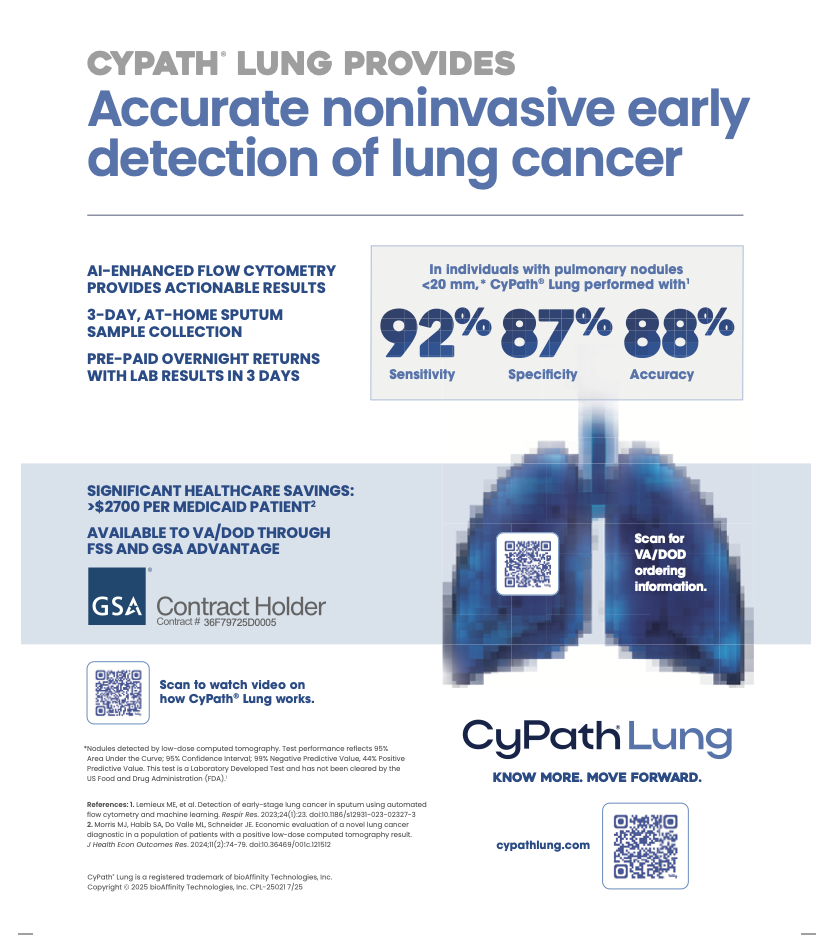
Sputum consists of a complex mixture of mucus from the lungs and a variety of cell types including cancer cells if a tumor is present. Sputum can be collected non-invasively with no patient side-effects which makes it an ideal specimen for diagnostic purposes. Sputum cytology was for decades the only tool available to diagnose lung cancer. However, sputum cytology is labor-intensive and requires significant expertise to read the slides. Moreover, the inter-observer variability is high. Together, these features of sputum cytology make for a poorly performing diagnostic test. Current imaging technologies, such as low-dose computed tomography (LDCT), are sensitive enough that small tumors can be reliably identified. Although very sensitive, LDCT has a high false positive rate, resulting in patients undergoing risky and expensive procedures such as bronchoscopy and lung biopsy to exclude a diagnosis of lung cancer. There is a need for an independent, less invasive test to aid in the diagnosis of lung cancer. Flow cytometry can rapidly analyze sputum cells and provide a platform to develop a novel diagnostic test for lung cancer.
Sputum samples were obtained from 30+ pack-year smokers at high risk of developing lung cancer. Study patients were asked to provide a sputum sample with the help of an acapella® assist device over 3 consecutive days. On the third day, the sputum sample was sent overnight to the laboratory. Upon arrival, sputum samples were liquefied using a mixture of N-acetyl-L-cysteine (NAC) and dithiothreitol (DTT), neutralized with HBSS and filtered through a cell strainer to create a single cell suspension. Dissociated cells were counted and viability was determined using trypan blue. On average, we obtained more than 20 million cells with greater than 65% viability and an average of 20% squamous epithelial cell (SEC) contamination. Dissociated sputum cells were labeled with a viability dye (FVS510) and a panel of markers to identify leukocyte and epithelium populations: CD45, CD66b, CD3, CD19, CD206, EpCAM and pan-cytokeratin. Cells were fixed with paraformaldehyde and analyzed using the BD LSRII flow cytometer. After excluding debris and dead cells, including SECs, we were able to identify reproducible signatures for both the CD45+ leukocyte and CD45– epithelial populations. CD206 served as the quality control marker as analysis of cytocentrifuge slides from cell sorting of the live CD45+CD206++ population confirmed these cells to be alveolar macrophages. Since alveolar macrophages only reside in the lung, CD206 can identify cells originating from the lung to confirm the sample origin.
We chose to investigate whether sputum can be analyzed on a flow cytometry platform analogous to its use for the diagnosis of hematologic malignancies. Our data reveals that flow cytometers can analyze samples isolated from sputum in a high-throughput manner that can further be standardized for lung cancer diagnostics.